







End-to-end solutions from study design, sample processing and analysis, to expert insights.
Secure and compliant cloud-based platform.
Regulatory compliant, ISO certified service provider with global recognition.
Customisable reporting from industry-standard metrics to validated, disease-specific signatures with deeper functional insights.
We provide an end-to-end sample collection, processing and data analytics service to generate clinical insights that improve drug development success rate and extend its commercial lifetime.

Patient Enrichment through Therapeutic Response Prediction
The response to immune checkpoint inhibitors is generally low in various cancers. Through KEYSTONE™, we discovered biomarkers that are highly predictive of clinical response. When deployed for patient enrichment before intervention, this can improve the therapeutic response rate from 50% to 96% in a patient cohort.
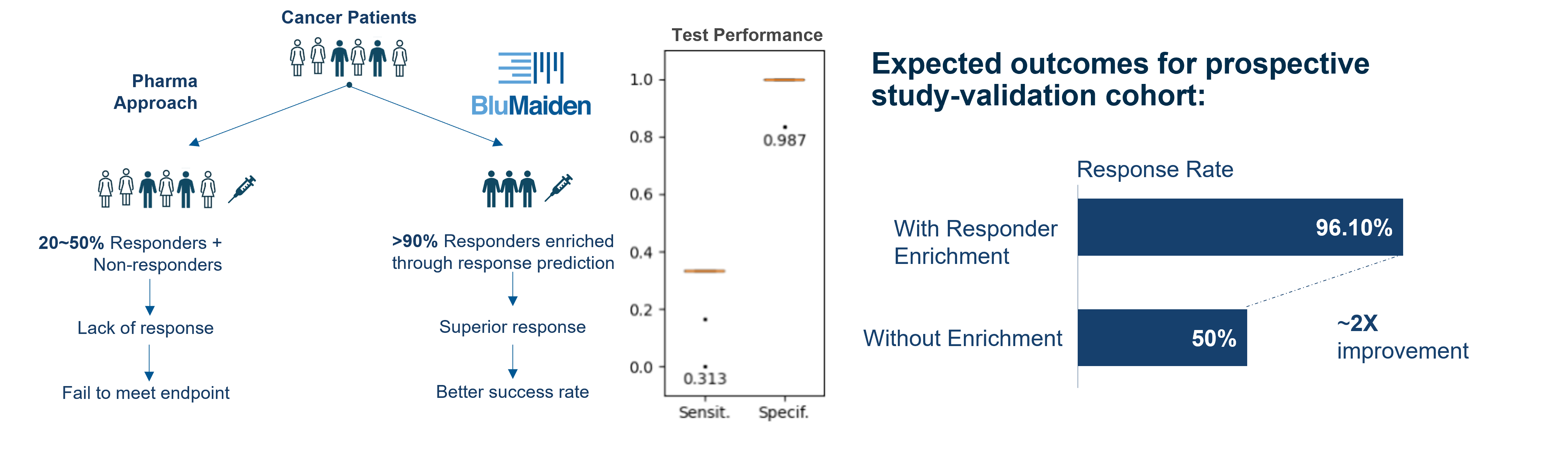
Patient Identification for Hard-to-Diagnose Indication
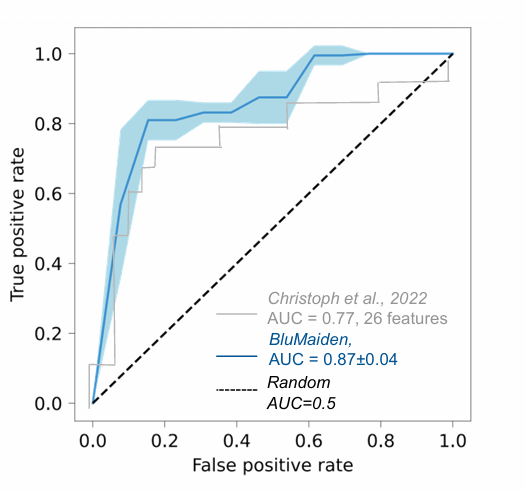
Alzheimer’s disease is hard to diagnose early as many conditions leads to dementia—its major symptom.
KEYSTONE™ discovered non-invasive biomarkers to aid the diagnosis of Alzheimer’s disease, facilitating trial recruitment screening, increases likelihood to include early disease stage participants.
Our diagnosis test generated superior performance (blue) compared to published benchmarks (grey).
Deep expertise in microbiome science, computational biology, machine learning and clinical trials.
Unique and highly-curated database of 4300+ globally-diverse reference data.
Valuable insights with significant clinical utility. We provide actionable understanding that accelerates trials and product development.




If you have a general question for us or want to explore a partnership opportunity, then please reach out to us here…